Introduction: Overweight & obesity represent prognostic factors for survival in multiple cancer entities, but the association between body mass index (BMI) & survival is not consistent across all cancer types. In acute myeloid leukemia (AML) data on the prognostic impact of diagnostic nutritional status remain inconsistent & in patients (pts) with hematologic malignancies, obesity prior to hematopoietic stem cell transplantation (HSCT) has been linked to inferior outcomes. Therefore, obesity has been included as one risk factor into the hematopoietic cell transplant comorbidity index (HCT-CI), which predicts non-relapse mortality (NRM) & overall survival (OS) in older HSCT pts. Additionally, AML pts often suffer from weight loss during therapy, which might also impair survival. Here we investigated the prognostic impact of the nutritional status at diagnosis & weight changes during therapy in AML pts receiving a HSCT.
Methods: We analyzed 662 AML pts who underwent allogeneic HSCT (median age 59.4, range 16.3-74.9 years) in complete remission (CR, 68%), CR with incomplete peripheral recovery (CRi, 14%) or active disease (18%) after non-myeloablative (59%), reduced intensity (15%) or myeloablative (26%) conditioning. Donors were matched related (20%), matched unrelated (59%), mismatched (19%) or haploidentical (2%). Comorbidities were assessed by the HCT-CI. The body mass index (BMI; kg/m2) at diagnosis, prior to HSCT & the BMI difference (∆BMI = BMIHSCT - BMIDx) were evaluated. For ∆BMI a cut-point of -2 was determined applying the R package "OptimalCutpoints" & divided pts into two groups with unchanged/increased BMI (∆BMI ≤-2, 57%) & decreased BMI (∆BMI >-2, 43%). AML disease risk was assessed according to European LeukemiaNet (ELN) 2017 classification & was 25% favorable, 30% intermediate & 45% adverse. Median follow up after HSCT was 3.1 years.
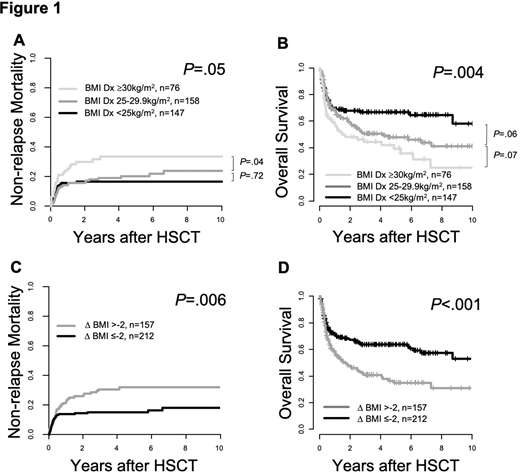
Results: The median BMI at AML diagnosis was significantly higher than at HSCT (median 25.8 vs 24.7 kg/m2, P<.001). According to WHO classification, at diagnosis vs HSCT 39% vs 53% of pts were under-/normal weight (BMI <25 kg/m2), 42% vs 35% were overweight (BMI 25-29.9 kg/m2)& 20% vs 12% were obese (BMI ≥30 kg/m2). Pts with ∆BMI >-2 were older (P=.002) & more likely to have de novo AML (P<.001) but did not vary regarding HCT-CI (P=.19) or pre-HSCT remission status (P=.99). Compared to non-obese pts, obese pts at diagnosis had higher NRM (P=.05) & shorter OS (P=.004, Figure 1A, B), while no significant prognostic impact was found for BMI at HSCT (NRM P=.15, OS P=.10). Weight loss (∆BMI >-2) between diagnosis & HSCT was a strong predictor for higher NRM (P=.006) & shorter OS (P<.001, Figure 1C, D). In multivariate analyses, ∆BMI >-2 remained significant for higher NRM (Hazard ratio 1.23, P=.008) after adjustment for donor type & for shorter OS (Odds ratio 0.82, P=.001) after adjustment for ELN risk, age & remission status at HSCT. Analyzing the three ELN risk groups separately, the prognostic impact of ∆BMI >-2 was particularly seen in ELN favorable (NRM P=.09, OS P=.02) & intermediate (NRM P=.02, OS P=.002) but not in ELN adverse risk pts (NRM P=.41, OS P=.20). ∆BMI >-2 was also a significant prognostic factor in pts transplanted in CR/CRi (NRM P=.01, OS P<.001) but not in the high-risk population of pts transplanted with active disease (NRM P=.15, OS P=.10). When we analyzed the prognostic impact of weight changes depending on the BMI category at diagnosis, we observed that weight loss (∆BMI >-2) had a prognostic impact in under-/normal weight (NRM P=.08, OS P=.007) & overweight (NRM P=.10, OS P=0.09), but not in obese pts (NRM P=.81, OS P=.70).
Conclusion: Obesity at diagnosis & weight loss (∆BMI >-2) associated with adverse outcomes due to higher NRM in AML pts undergoing HSCT. The prognostic impact of weight loss between diagnosis & HSCT represented a strong, potentially modifiable & independent risk factor & was particularly seen in non-high-risk pts. In high-risk pts with ELN adverse risk or with active disease at HSCT, weight loss did not impact outcomes, most likely due to the aggressive phenotype of the underlying AML. The data indicates that nutritional dynamics in AML pts destined for HSCT should be monitored & supportive therapy adjusted accordingly to improve outcomes.
Vucinic:Celgene: Honoraria. Niederwieser:Novartis: Speakers Bureau; Cellectis: Membership on an entity's Board of Directors or advisory committees; Amgen: Speakers Bureau; Daiichi: Research Funding. Platzbecker:Janssen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Geron: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Jentzsch:Novartis: Honoraria; JAZZ Pharmaceuticals: Honoraria. Schwind:Pfizer: Honoraria; Novartis: Honoraria, Research Funding; JAZZ Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal